Abstract
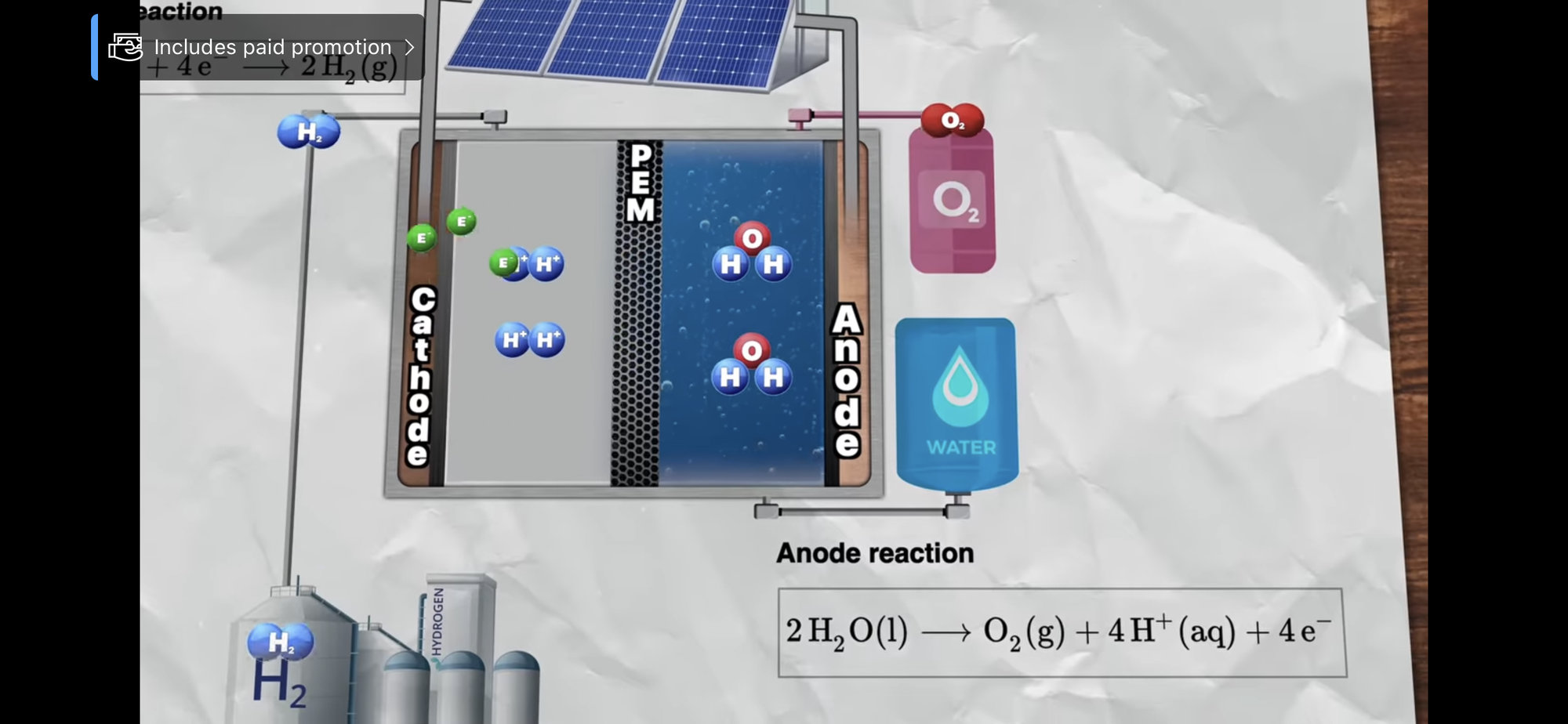
Proton exchange membrane water electrolysis (PEMWE) is a key technology for future sustainable energy systems. Proton exchange membrane (PEM) electrolysis cells use iridium, one of the scarcest elements on earth, as catalyst for the oxygen evolution reaction. In the present study, the expected iridium demand and potential bottlenecks in the realization of PEMWE for hydrogen production in the targeted GW a−1 scale are assessed in a model built on three pillars: (i) an in-depth analysis of iridium reserves and mine production, (ii) technical prospects for the optimization of PEM water electrolyzers, and (iii) PEMWE installation rates for a market ramp-up and maturation model covering 50 years. As a main result, two necessary preconditions have been identified to meet the immense future iridium demand: first, the dramatic reduction of iridium catalyst loading in PEM electrolysis cells and second, the development of a recycling infrastructure for iridium catalysts with technical end-of-life recycling rates of at least 90%.